Order/Disorder in Minerals and Ceramic Materials
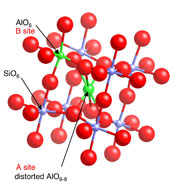
Most minerals in nature are solid solutions. Their properties, such as the configurational entropy or the free energy of mixing needed to calculate phase equilibria, thus depend on the details of the distribution of cations and anions on available sites: is this truly random, or is there some type of short-range ordering or clustering? Related questions can be asked about coupled substitutions, for example a commonly proposed mechanism of dissolution of rare earth elements (REE) in zircon: REE(3+) + P(5+) = Zr(4+) + Si(4+). If this occurs, are the REE and P associated to produce local charge balance, or are they distributed throughout the structure (entropy wins at high temperature...)? Even in the pure end-members of minerals such as the polymorphs of MgSiO3 so critical in the mantle, site exchange could occur, for example that of Mg and Si among octahedral and tetrahedral sites at high pressures and temperatures.
Solid solutions are important in ceramic technology as well. A dramatic example is that of oxide-ion conductors such as yttrium-stabilized zirconia (YSZ), which forms the electrolyte of most solid-oxide fuel cells and of most oxygen sensors in automobile engines. The distribution of Y3+ (and more exotic) cations in the crystalline lattice controls the mobility of oxygen defects and hence the critical electrical properties.
Conventional methods of determining crystal structure, such as X-ray diffraction, generally reveal only the long-range average structure, and often thus cannot give the details of energetically critical short range order/disorder. Again, NMR spectroscopy can provide unique constraints on such problems in many systems.
Current Projects
- Order/disorder in high-pressure silicate minerals
- Cation disorder and vacancies in ionicly conducting ceramics
- Cation order/disorder and substitution mechanisms in oxides with magnetic cations